Welcome to the official website of Shandong Eagle Pharma Machinery Co.,Ltd.!
Service Hotline:+86-536-8800237
Product Center
Contact Us
Address: Block B, Headquarters Base ABP, No.2600, Zhuangjian Road.Weifang, Shandong, P.R.C.
Tel: +86-536-8800237
Email: export@sdwemac.com
WhatsApp: Naomi: 86 15315252862
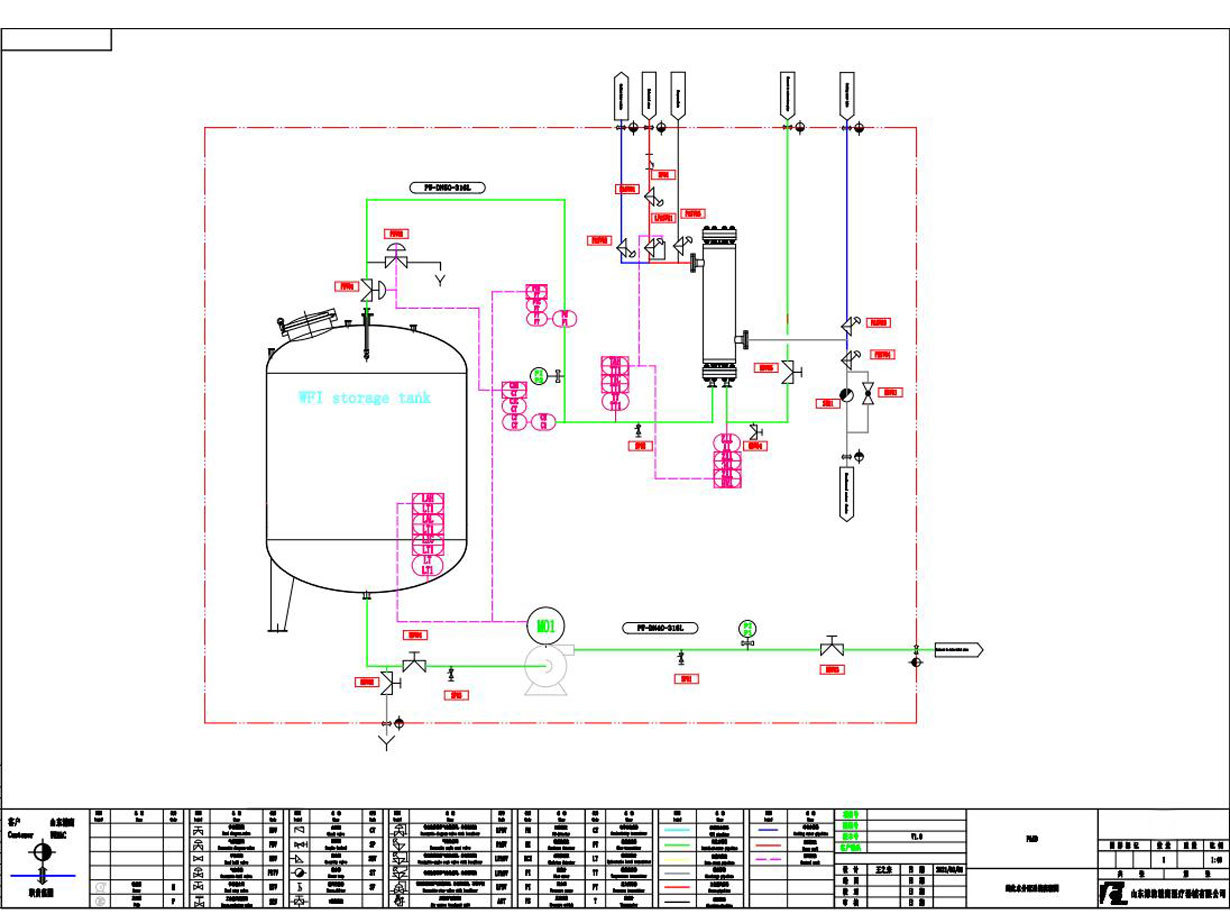
WFI Distribution Module
Design according to the actual needs of the customer’s site and the disinfection requirements, with GMP, ISPE engineering guidelines and FDA specifications as the design concept, rational use of space, convenient use and operation, and selection of high-quality clean pipe fittings. So that the design and installation are free of dead ends and no contamination. The reflux control is reasonable and fully meets the user's requirements and verification requirements.
Keywords:
category:
Details description
The requirements of WFI Distribution Module
1. The material in contact with water for injection must be high-quality low-carbon stainless steel (such as 316L stainless steel) or other materials that have been verified to not pollute the water. Medical purified water equipment should be cleaned regularly, and the cleaning effect should be verified.
2. The storage period of water for injection should not be longer than 24 hours, and the storage tank should be made of stainless steel or other materials that have been proven to be non-toxic, corrosion-resistant and do not exude polluting ions. To protect the vent, a hydrophobic sterilizing filter that does not shed fibers should be installed. The inner wall of the storage tank should be smooth, and there should be no dead ends and trachoma in the joints and welds. Sensors that display parameters such as liquid level, temperature and pressure that will not form stagnant water pollution should be used. The storage tank should be cleaned, disinfected and sterilized regularly, and the effect of cleaning and sterilization should be verified.
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Packing and shipping




FAQ






Related Products












