Welcome to the official website of Shandong Eagle Pharma Machinery Co.,Ltd.!
Service Hotline:+86-536-8800237
Product Center
Contact Us
Address: Block B, Headquarters Base ABP, No.2600, Zhuangjian Road.Weifang, Shandong, P.R.C.
Tel: +86-536-8800237
Email: export@sdwemac.com
WhatsApp: Naomi: 86 15315252862
WFI machine
WEMAC customized optimization design according to the raw water quality report,effluent quality requirements and the actual needs of customers, and customer can also choose the standardized design from WEMAC. The output water quality can meet the requirements of Chinese Pharmacopoeia(CP2020), United States Pharmacopoeia (USP43) and European Pharmacopoeia (EP10) for purified water or high purity water according to different system configurations.
Keywords:
category:
Details description
Since water for injection is the most widely used raw and auxiliary material in the pharmaceutical industry, to ensure the quality of drugs, the quality of WFI must be ensured first. Starting from this goal, countries have made very clear regulations on the production methods of water for injection according to their actual conditions.
USP 24 stipulates that WFI must be purified by distillation or reverse osmosis for drinking water that meets the legal requirements of the US Environmental Protection Association or the European Community or Japan.
The European Pharmacopoeia (1997 edition) stipulates that "water for injection is obtained by appropriate distillation of drinking water or pure water that meets legal standards".
According to the Chinese Pharmacopoeia, this product (WFI) is water obtained by distillation of purified water.
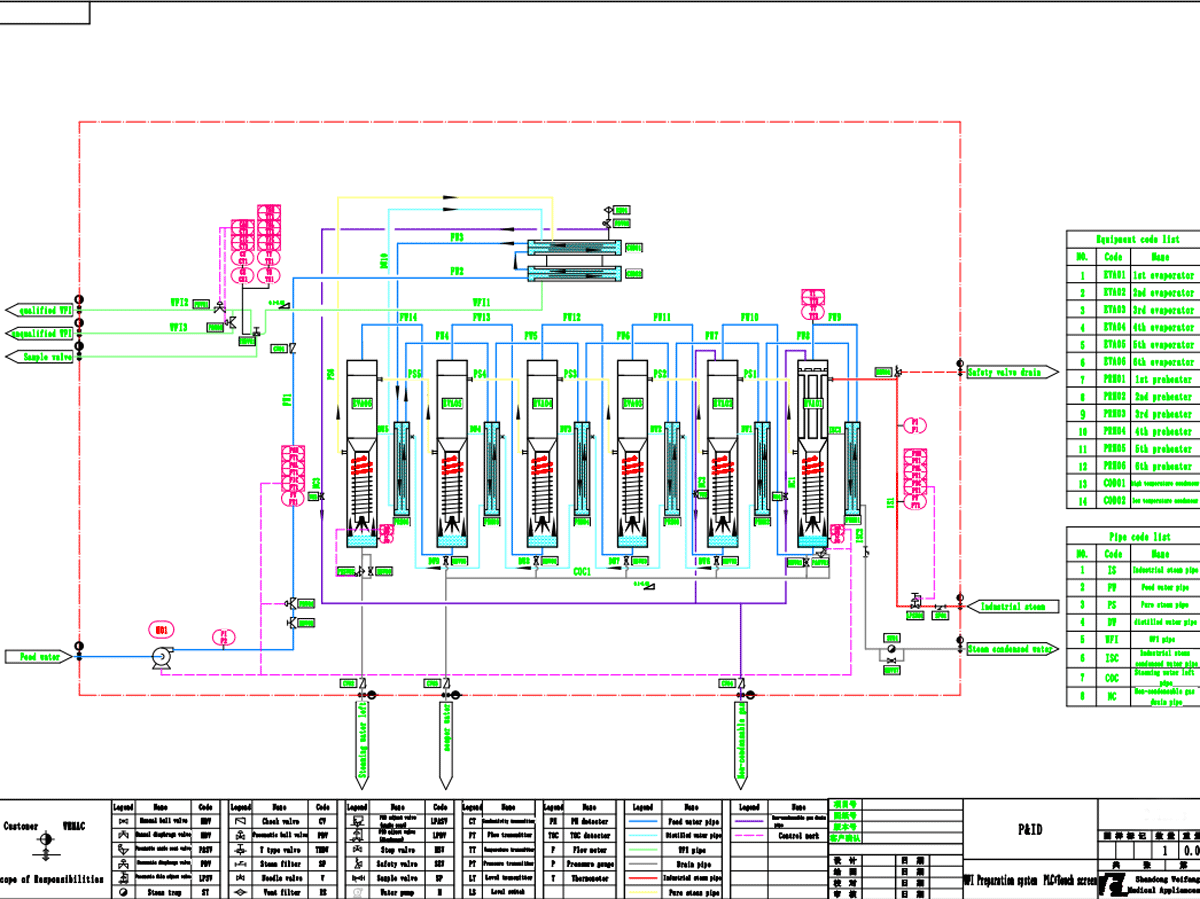
It can be seen that distillation is the preferred method for preparing water for injection recognized by all countries in the world, with a long history and reliable results. The distillation process is a heat treatment process. Taking a WFI machine as an example, the temperature of the first evaporation column is as high as 150 °C, and the last column is at 110 °C. The distillation process is also a process of disinfection and sterilization. Fresh water for injection in the distilled water machine It is a well-known fact that no bacteria are always detected by sampling at the outlet. The stability, reliability and safety of the system and water quality are the main reasons why pharmaceutical companies choose distillation as the preferred preparation method of water for injection.
| Model | Capacity | Industry steam consumption(L/h) | Feed water consumption(L/h) | Cooling water consumption(L/h) | Dimensions(mm) |
| LDS100-4 | ≥100 | 30 | 110 | 80 | 1065*540*1900 |
| LDS300-5 | ≥300 | 80 | 330 | 170 | 1610*650*2300 |
| LDS500-4 | ≥500 | 150 | 550 | 550 | 1540*770*3150 |
| LDS500-5 | ≥1000 | 115 | 550 | 190 | 1900*770*3150 |
| LDS1000-5 | ≥1000 | 250 | 1100 | 350 | 2200*1000*3400 |
| LDS1000-6 | ≥2000 | 210 | 1100 | 0 | 2830*1000*3400 |
| LDS2000-5 | ≥2000 | 520 | 2200 | 750 | 3000*1200*3600 |
| LDS2000-6 | ≥2000 | 440 | 2200 | 0 | 3510*1200*3600 |
| LDS3000-6 | ≥3000 | 670 | 3300 | 0 | 3600*1200*3750 |
| LDS4000-6 | ≥4000 | 900 | 4400 | 0 | 4080*1250*4000 |
| LDS5000-6 | ≥5000 | 1130 | 5500 | 0 | 4470*1450*4250 |
| LDS6000-6 | ≥6000 | 1350 | 6600 | 0 | 4470*1450*4500 |

 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Packing and shipping




FAQ






Related Products












