Welcome to the official website of Shandong Eagle Pharma Machinery Co.,Ltd.!
Service Hotline:+86-536-8800237
Product Center
Contact Us
Address: Block B, Headquarters Base ABP, No.2600, Zhuangjian Road.Weifang, Shandong, P.R.C.
Tel: +86-536-8800237
Email: export@sdwemac.com
WhatsApp: Naomi: 86 15315252862
Water system for pharmaceutical
WEMAC customized optimization design according to the raw water quality report,effluent quality requirements and the actual needs of customers, and customer can also choose the standardized design from WEMAC. The output water quality can meet the requirements of Chinese Pharmacopoeia(CP2020), United States Pharmacopoeia (USP43) and European Pharmacopoeia (EP10) for purified water or high purity water according to different system configurations.
Keywords:
category:
Details description
Water system for pharmaceutical is a water supply system specially designed for the water used in various drug production processes. Its process design is flexible. Different types of drug production may have different requirements for water quality. Therefore, customers provide their own water data to design purified water equipment.
1. The process of preparing in vitro reagent water by water system for pharmaceutical
The mechanism of the main desalination part of the RO membrane element is similar to that of the semi-permeable membrane, which can selectively permeate the ions in the water. In the natural state, the semi-permeable membrane (RO membrane) selectively permeates the solvent (water) from the low concentration side. The high-concentration side conducts natural infiltration, and achieves natural infiltration balance under the formation of a certain osmotic pressure difference. When external pressure is applied to the high-concentration side, the solvent on the high-concentration side overcomes the natural osmotic pressure and the natural liquid level height difference, so that water molecules are transported from the water. The high concentration side is reverse osmosis to the low concentration side. RO membrane separation systems operate in a completely different way than traditional filtration systems. When the traditional filtration system is running, all the water passes through the filter layer of the filter. When the interception capacity is reduced to a certain limit, the intercepted pollutants are removed from the filter layer by the backwashing operation of the equipment. When the RO system is running, a part of the water flow in the raw water permeates the membrane in a direction perpendicular to the membrane surface, while the other non-permeable part of the water flow flows in a direction parallel to the membrane surface, which belongs to cross-flow filtration in technology. category.
2. Process characteristics of in vitro reagent water system for pharmaceutical
1. The water system for pharmaceutical can operate continuously to produce water, the system is simple, the operation is convenient, and the quality of the product water is stable.
2. The workload of operation and maintenance and equipment maintenance is very small.
3. The purified water equipment occupies less area and requires less space.
4. Personalized design in line with local water quality to meet the needs in an all-round way.
| Name | Description | |||||
| Pre-treatment system | Pretreatment process contains traditional processes, such as Feed water storage tank,Heat exchanger heating device,Mechanical filtrater, activated carbon filtrater, softener,Precision Filter, dosing device, as well as ultra-filtration, residual chlorine removal UV, micro-filtration, etc. | |||||
| RO2 system | Based on demands of raw water quality conditions and terminal water,RO2 can be used as terminal processing of PW generation system. If alkalinity of raw water is on the high side, degassing device can be chosen. The water quality conforms to requirements of Chinese pharmacopoeia, European pharmacopoeia and United States pharmacopoeia. |
|||||
| RO1+EDI system | Based on demands of raw water quality conditions and terminal water, RO1 +EDI can be used as terminal processing of PW generation system. Quality of water produced by RO1 + EDI is stable, only slightly affected by fluctuation of raw water quality. The water quality conforms to requirements of Chinese pharmacopoeia, European pharmacopoeia and United States pharmacopoeia. |
|||||
| RO2+EDI system | Adopting RO2+EDI system as terminal processing of PW generation system, produced water will only be slightly affected by fluctuation of raw water quality. Quality of produced water is stable and highly above requirements of Pharmacopoeia. | |||||
| Residual chlorine removal UV system | Using residual chlorine removal UV system in pretreatment, instead of activated carbon filter. At the same time of efficient removal of residual chlorine, system can completely inactivate raw water microorganism, ensuring safety of microorganism from the source and improving quality of supply water. It supports online UV intensity monitoring and dosage display, which can accurately predict dechlorination effect and completely avoid troubles from activated carbon filter. |
|||||
 |
Can choose 304 or 316L material, and use professional equipment and equipment to strictly inspect the quality;
|
 |
Online monitoring of the water quality meets customer needs;
|
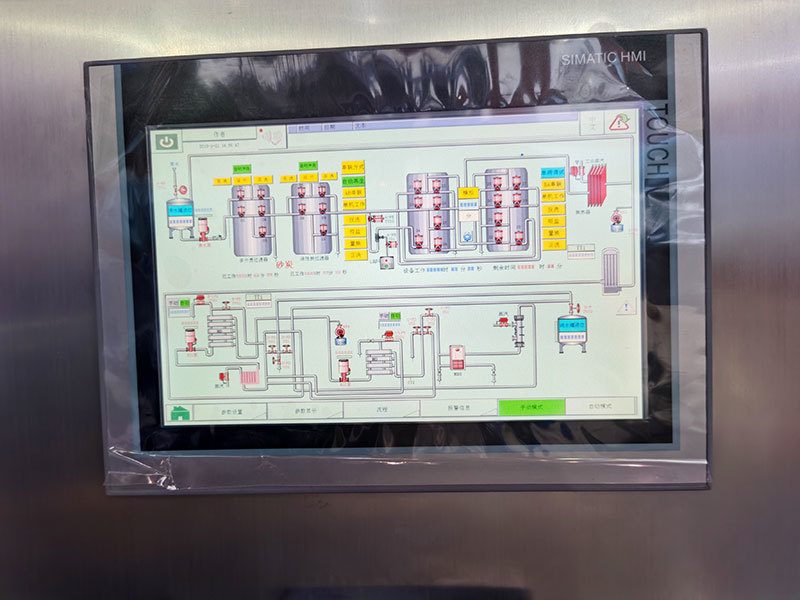 |
Has three levels of management authority, followed by operator, administrator, and senior administrator. Each login account has a corresponding login password (the password can be modified), and the system will automatically log out after 5 minutes; prevent unauthorized personnel from entering the system from misoperation, and the parameters can be restored to factory settings;
|
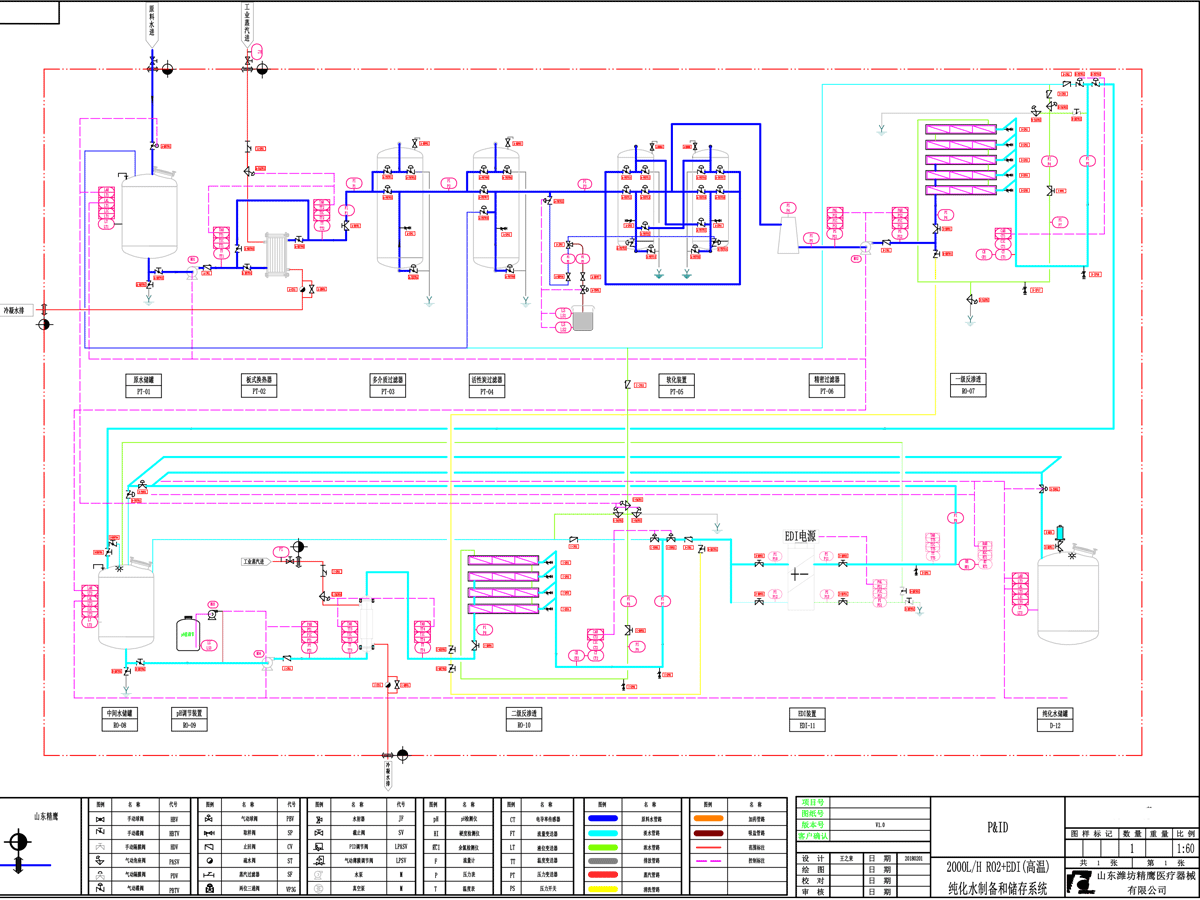
 |
 |
 |
 |
 |
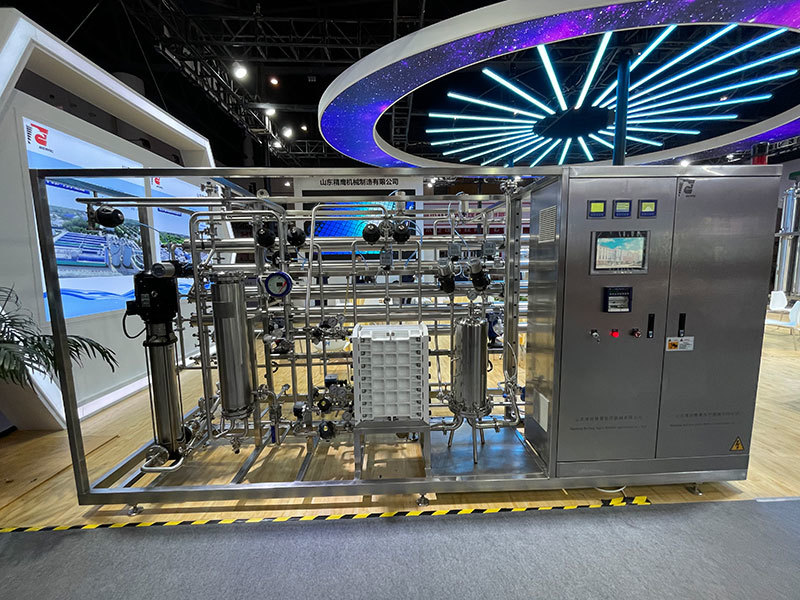 |
 |
 |
 |
 |

 |
 |
 |
 |
 |
 |
 |
 |
Packing and shipping




FAQ






Related Products












